Search
- Page Path
- HOME > Search
- Calcium & Bone Metabolism
- Bone Mineral Density Screening Interval and Transition to Osteoporosis in Asian Women
- Hyunju Park, Heera Yang, Jung Heo, Hye Won Jang, Jae Hoon Chung, Tae Hyuk Kim, Yong-Ki Min, Sun Wook Kim
- Endocrinol Metab. 2022;37(3):506-512. Published online June 9, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1429
- 3,068 View
- 103 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
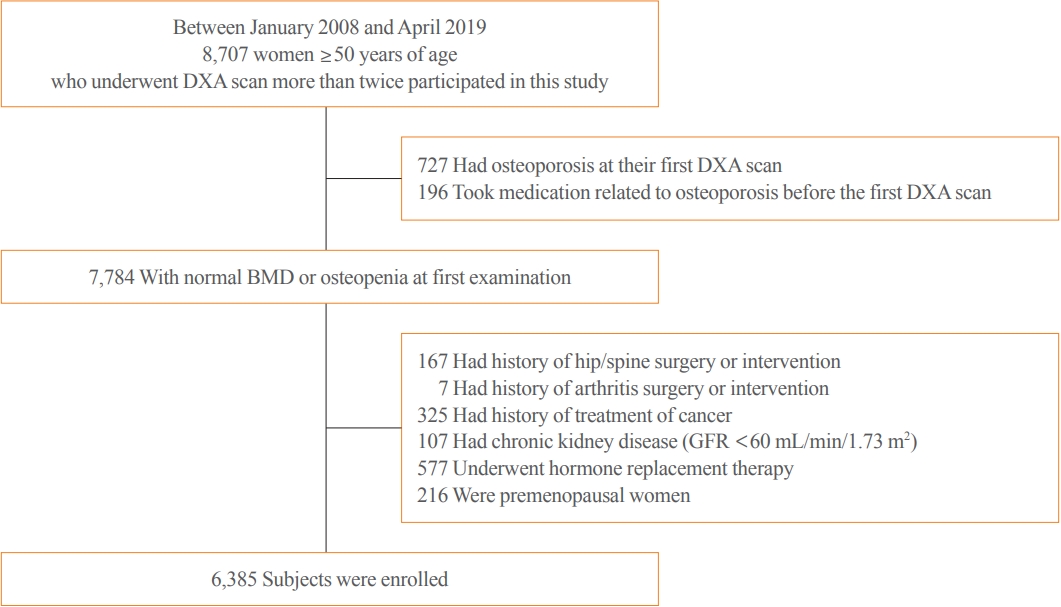
Bone mineral density (BMD) testing is indicated for women aged 65 years, but screening strategies for osteoporosis are controversial. Currently, there is no study focusing on the BMD testing interval in Asian populations. The current study aimed to evaluate the estimated time interval for screening osteoporosis.
Methods
We conducted a study of 6,385 subjects aged 50 years and older who underwent dual-energy X-ray absorptiometry screening more than twice at Samsung Medical Center as participants in a routine health checkup. Subjects were divided based on baseline T-score into mild osteopenia (T-score, <–1.0 to >–1.5), moderate osteopenia (T-score, ≤–1.5 to >–2.0), and severe osteopenia (T-score, ≤–2.0 to >–2.5). Information about personal medical and social history was collected by a structured questionnaire.
Results
The adjusted estimated BMD testing interval for 10% of the subjects to develop osteoporosis was 13.2 years in mild osteopenia, 5.0 years in moderate osteopenia, and 1.5 years in severe osteopenia.
Conclusion
Our study provides extended information about BMD screening intervals in Asian female population. Baseline T-score was important for predicting BMD screening interval, and repeat BMD testing within 5 years might not be necessary in mild osteopenia subjects. -
Citations
Citations to this article as recorded by- Effects of Bazedoxifene/Vitamin D Combination Therapy on Serum Vitamin D Levels and Bone Turnover Markers in Postmenopausal Women with Osteopenia: A Randomized Controlled Trial
Chaiho Jeong, Jeonghoon Ha, Jun-Il Yoo, Young-Kyun Lee, Jung Hee Kim, Yong-Chan Ha, Yong-Ki Min, Dong-Won Byun, Ki-Hyun Baek, Ho Yeon Chung
Journal of Bone Metabolism.2023; 30(2): 189. CrossRef - Bone-modifying agents for non–small-cell lung cancer patients with bone metastases during the era of immune checkpoint inhibitors: A narrative review
Jinyoung Kim, Chaiho Jeong, Jeongmin Lee, Jeonghoon Ha, Ki-Hyun Baek, Seohyun Kim, Tai Joon An, Chan Kwon Park, Hyoung Kyu Yoon, Jeong Uk Lim
Seminars in Oncology.2023; 50(3-5): 105. CrossRef
- Effects of Bazedoxifene/Vitamin D Combination Therapy on Serum Vitamin D Levels and Bone Turnover Markers in Postmenopausal Women with Osteopenia: A Randomized Controlled Trial

- Bone Metabolism
- Preventing Rebound-Associated Fractures after Discontinuation of Denosumab Therapy: A Position Statement from the Health Insurance Committee of the Korean Endocrine Society
- Bu Kyung Kim, Chong Hwa Kim, Yong-Ki Min
- Endocrinol Metab. 2021;36(4):909-911. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1193

- 3,480 View
- 167 Download
- 6 Web of Science
- 6 Crossref
-
 PDF
PDF PubReader
PubReader  ePub
ePub -
Citations
Citations to this article as recorded by- Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study
Chaiho Jeong, Jeongmin Lee, Jinyoung Kim, Jeonghoon Ha, Kwanhoon Jo, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Tae-Seo Sohn, Ki-Ho Song, Moo Il Kang, Ki-Hyun Baek
Endocrinology and Metabolism.2023; 38(2): 260. CrossRef - Raloxifene Use After Denosumab Discontinuation Partially Attenuates Bone Loss in the Lumbar Spine in Postmenopausal Osteoporosis
Namki Hong, Sungjae Shin, Seunghyun Lee, Kyoung Jin Kim, Yumie Rhee
Calcified Tissue International.2022; 111(1): 47. CrossRef - Effect of follow-up raloxifene therapy after denosumab discontinuation in postmenopausal women
J. Ha, J. Kim, C. Jeong, Y. Lim, M. K. Kim, H.-S. Kwon, K.-H. Song, M. I. Kang, K.-H. Baek
Osteoporosis International.2022; 33(7): 1591. CrossRef - Discontinuing Denosumab: Can It Be Done Safely? A Review of the Literature
Wei Lin Tay, Donovan Tay
Endocrinology and Metabolism.2022; 37(2): 183. CrossRef - Real-World Safety and Effectiveness of Denosumab in Patients with Osteoporosis: A Prospective, Observational Study in South Korea
Yumie Rhee, Dong-Gune Chang, Jeonghoon Ha, Sooa Kim, Yusun Lee, Euna Jo, Jung-Min Koh
Endocrinology and Metabolism.2022; 37(3): 497. CrossRef - Long-term consequences of osteoporosis therapy with denosumab
Francisco Bandeira, Lucian Batista de Oliveira, John P. Bilezikian
Archives of Endocrinology and Metabolism.2022; 66(5): 717. CrossRef
- Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study

- Clinical Study
- Romosozumab in Postmenopausal Korean Women with Osteoporosis: A Randomized, Double-Blind, Placebo-Controlled Efficacy and Safety Study
- Ki-Hyun Baek, Yoon-Sok Chung, Jung-Min Koh, In Joo Kim, Kyoung Min Kim, Yong-Ki Min, Ki Deok Park, Rajani Dinavahi, Judy Maddox, Wenjing Yang, Sooa Kim, Sang Jin Lee, Hyungjin Cho, Sung-Kil Lim
- Endocrinol Metab. 2021;36(1):60-69. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2020.848
- 6,817 View
- 390 Download
- 7 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
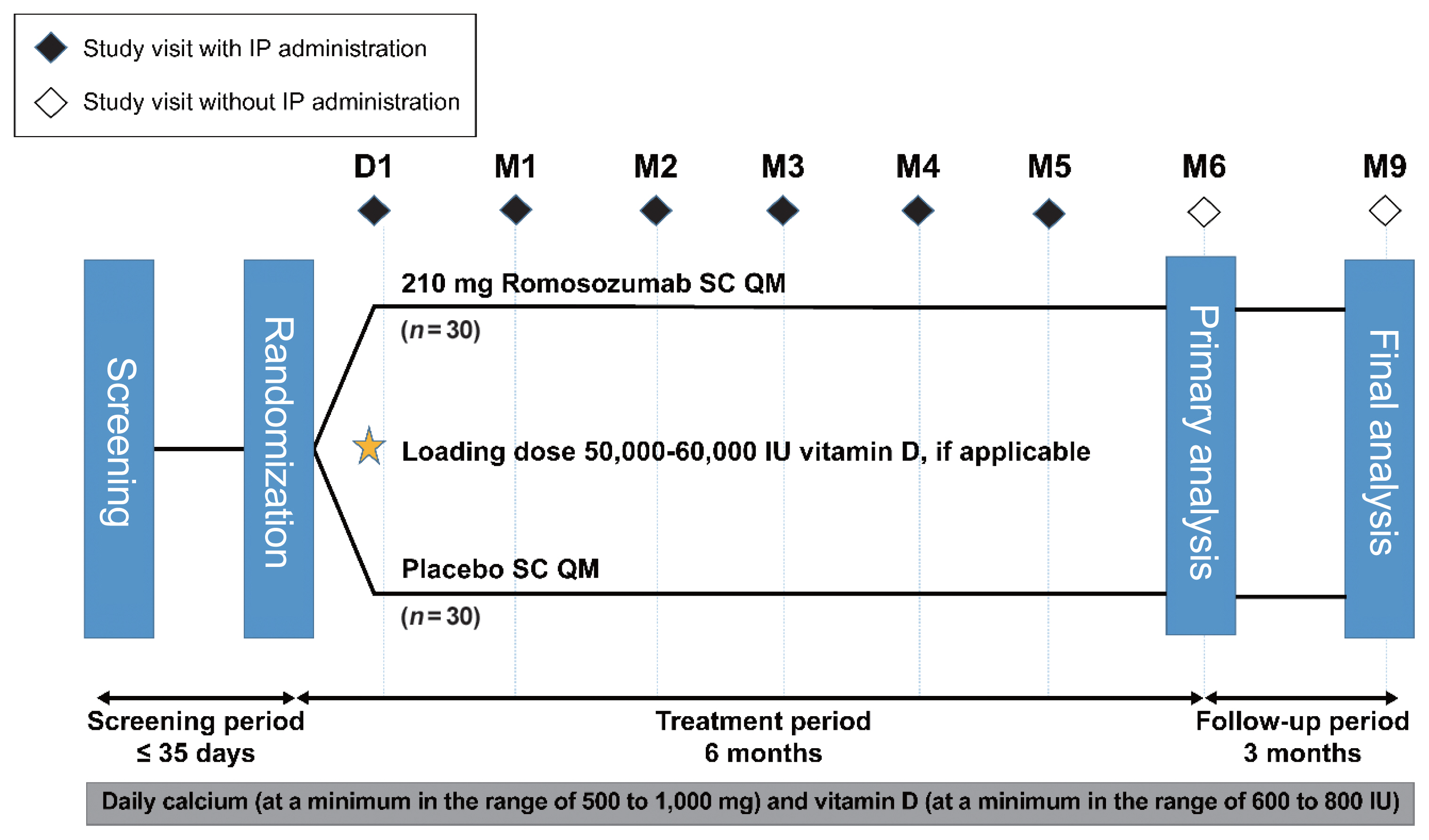
This phase 3 study evaluated the efficacy and safety of 6-month treatment with romosozumab in Korean postmenopausal women with osteoporosis.
Methods
Sixty-seven postmenopausal women with osteoporosis (bone mineral density [BMD] T-scores ≤–2.5 at the lumbar spine, total hip, or femoral neck) were randomized (1:1) to receive monthly subcutaneous injections of romosozumab (210 mg; n=34) or placebo (n=33) for 6 months.
Results
At month 6, the difference in the least square (LS) mean percent change from baseline in lumbar spine BMD (primary efficacy endpoint) between the romosozumab (9.5%) and placebo (–0.1%) groups was significant (9.6%; 95% confidence interval, 7.6 to 11.5; P<0.001). The difference in the LS mean percent change from baseline was also significant for total hip and femoral neck BMD (secondary efficacy endpoints). After treatment with romosozumab, the percent change from baseline in procollagen type 1 N-terminal propeptide transiently increased at months 1 and 3, while that in C-terminal telopeptide of type 1 collagen showed a sustained decrease. No events of cancer, hypocalcemia, injection site reaction, positively adjudicated atypical femoral fracture or osteonecrosis of the jaw, or positively adjudicated serious cardiovascular adverse events were observed. At month 9, 17.6% and 2.9% of patients in the romosozumab group developed binding and neutralizing antibodies, respectively.
Conclusion
Treatment with romosozumab for 6 months was well tolerated and significantly increased lumbar spine, total hip, and femoral neck BMD compared with placebo in Korean postmenopausal women with osteoporosis (ClinicalTrials.gov identifier NCT02791516). -
Citations
Citations to this article as recorded by- A pharmacovigilance analysis of FDA adverse event reporting system events for romosozumab
Zepeng Chen, Ming Li, Shuzhen Li, Yuxi Li, Junyan Wu, Kaifeng Qiu, Xiaoxia Yu, Lin Huang, Guanghui Chen
Expert Opinion on Drug Safety.2023; 22(4): 339. CrossRef - Evaluation of the efficacy and safety of romosozumab (evenity) for the treatment of osteoporotic vertebral compression fracture in postmenopausal women: A systematic review and meta‐analysis of randomized controlled trials (CDM‐J)
Wenbo Huang, Masashi Nagao, Naohiro Yonemoto, Sen Guo, Takeshi Tanigawa, Yuji Nishizaki
Pharmacoepidemiology and Drug Safety.2023; 32(6): 671. CrossRef - Efficacy and Cardiovascular Safety of Romosozumab: A Meta-analysis and Systematic Review
Seo-Yong Choi, Jeong-Min Kim, Sang-Hyeon Oh, Seunghyun Cheon, Jee-Eun Chung
Korean Journal of Clinical Pharmacy.2023; 33(2): 128. CrossRef - Clinical Studies On Romosozumab: An Alternative For Individuals With A High Risk Of Osteoporotic Fractures: A Current Concepts Review (Part I)
E. Carlos Rodriguez-Merchan, Alonso Moreno-Garcia, Hortensia De la Corte-Rodriguez
SurgiColl.2023;[Epub] CrossRef - Romosozumab in osteoporosis: yesterday, today and tomorrow
Dong Wu, Lei Li, Zhun Wen, Guangbin Wang
Journal of Translational Medicine.2023;[Epub] CrossRef - Efficacy and safety of anti-sclerostin antibodies in the treatment of osteoporosis: A meta-analysis and systematic review
Frideriki Poutoglidou, Efthimios Samoladas, Nikolaos Raikos, Dimitrios Kouvelas
Journal of Clinical Densitometry.2022; 25(3): 401. CrossRef - Benefits of lumican on human bone health: clinical evidence using bone marrow aspirates
Yun Sun Lee, So Jeong Park, Jin Young Lee, Eunah Choi, Beom-Jun Kim
The Korean Journal of Internal Medicine.2022; 37(4): 821. CrossRef - What is the risk of cardiovascular events in osteoporotic patients treated with romosozumab?
I. R. Reid
Expert Opinion on Drug Safety.2022; 21(12): 1441. CrossRef - Proxied Therapeutic Inhibition on Wnt Signaling Antagonists and Risk of Cardiovascular Diseases: Multi-Omics Analyses
Yu Qian, Cheng-Da Yuan, Saber Khederzadeh, Ming-Yu Han, Hai-Xia Liu, Mo-Chang Qiu, Jian-Hua Gao, Wei-Lin Wang, Yun-Piao Hou, Guo-Bo Chen, Ke-Qi Liu, Lin Xu, David Karasik, Shu-Yang Xie, Hou-Feng Zheng
SSRN Electronic Journal .2022;[Epub] CrossRef - Multi-Omics Analyses Identify Pleiotropy and Causality Between Circulating Sclerostin and Atrial Fibrillation
Yu Qian, Peng-Lin Guan, Saber Khederzadeh, Ke-Qi Liu, Cheng-Da Yuan, Ming-Yu Han, Hai-Xia Liu, Mo-Chang Qiu, Jian-Hua Gao, Wei-Lin Wang, Yun-Piao Hou, Guo-Bo Chen, Lin Xu, David Karasik, Shu-Yang Xie, sheng zhifeng, Hou-Feng Zheng
SSRN Electronic Journal .2022;[Epub] CrossRef
- A pharmacovigilance analysis of FDA adverse event reporting system events for romosozumab

- Clinical Study
- Triiodothyronine Levels Are Independently Associated with Metabolic Syndrome in Euthyroid Middle-Aged Subjects
- Hye Jeong Kim, Ji Cheol Bae, Hyeong Kyu Park, Dong Won Byun, Kyoil Suh, Myung Hi Yoo, Jae Hyeon Kim, Yong-Ki Min, Sun Wook Kim, Jae Hoon Chung
- Endocrinol Metab. 2016;31(2):311-319. Published online May 13, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.311
- 4,462 View
- 32 Download
- 26 Web of Science
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Recent studies have shown an association between thyroid hormone levels and metabolic syndrome (MetS) among euthyroid individuals; however, there have been some inconsistencies between studies. Here, we evaluated the relationship between thyroid hormone levels and MetS in euthyroid middle-aged subjects in a large cohort.
Methods A retrospective analysis of 13,496 euthyroid middle-aged subjects who participated in comprehensive health examinations was performed. Subjects were grouped according to thyroid stimulating hormone, total triiodothyronine (T3), total thyroxine (T4), and T3-to-T4 ratio quartile categories. We estimated the odds ratios (ORs) for MetS according to thyroid hormone quartiles using logistic regression models, adjusted for potential confounders.
Results Of the study patients, 12% (
n =1,664) had MetS. A higher T3 level and T3-to-T4 ratio were associated with unfavourable metabolic profiles, such as higher body mass index, systolic and diastolic blood pressure, triglycerides, fasting glucose and glycated hemoglobin, and lower high density lipoprotein cholesterol levels. The proportion of participants with MetS increased across the T3 quartile categories (P for trend <0.001) and the T3-to-T4 ratio quartile categories (P for trend <0.001). The multi-variate-adjusted OR (95% confidence interval) for MetS in the highest T3 quartile group was 1.249 (1.020 to 1.529) compared to the lowest T3 quartile group, and that in the highest T3-to-T4 ratio quartile group was 1.458 (1.141 to 1.863) compared to the lowest T3-to-T4 ratio quartile group, even after adjustment for potential confounders.Conclusion Serum T3 levels and T3-to-T4 ratio are independently associated with MetS in euthyroid middle-aged subjects. Longitudinal studies are needed to define this association and its potential health implications.
-
Citations
Citations to this article as recorded by- The effect of endocrine disrupting chemicals on the vitronectin-receptor (integrin αvβ3)-mediated cell adhesion of human umbilical vein endothelial cells
Maša Kenda, Urša Pečar Fonović, Janko Kos, Marija Sollner Dolenc
Toxicology in Vitro.2022; 79: 105275. CrossRef - Could the ketogenic diet induce a shift in thyroid function and support a metabolic advantage in healthy participants? A pilot randomized-controlled-crossover trial
Stella Iacovides, Shane K. Maloney, Sindeep Bhana, Zareena Angamia, Rebecca M. Meiring, Carla Pegoraro
PLOS ONE.2022; 17(6): e0269440. CrossRef - Mediation effects of thyroid function in the associations between phthalate exposure and lipid metabolism in adults
Han-Bin Huang, Po-Keng Cheng, Chi-Ying Siao, Yuan-Ting C. Lo, Wei-Chun Chou, Po-Chin Huang
Environmental Health.2022;[Epub] CrossRef - Cholinesterase homozygous genotype as susceptible biomarker of hypertriglyceridaemia for pesticide-exposed agricultural workers
Xingfan Zhou, Min Zhang, Yuqian Wang, Hailing Xia, Lijin Zhu, Guangyi Li, Li Rong, Huahuang Dong, Rui Chen, Shichuan Tang, Min Yu
Biomarkers.2021; 26(4): 335. CrossRef - Association between thyroid hormone and components of metabolic syndrome in euthyroid Korean adults
Kyung A. Shin, Eun Jae Kim
Medicine.2021; 100(51): e28409. CrossRef - Clinical Parameters Are More Likely to Be Associated with Thyroid Hormone Levels than with Thyrotropin Levels: A Systematic Review and Meta-Analysis
Stephen P. Fitzgerald, Nigel G. Bean, Henrik Falhammar, Jono Tuke
Thyroid.2020; 30(12): 1695. CrossRef - The role of thyroid hormone in metabolism and metabolic syndrome
Patrícia de Fátima dos Santos Teixeira, Patrícia Borges dos Santos, Carmen Cabanelas Pazos-Moura
Therapeutic Advances in Endocrinology and Metabolism.2020; 11: 204201882091786. CrossRef - Association between Abdominal Fat Distribution and Free Triiodothyronine in a Euthyroid Population
Xiaomin Nie, Yiting Xu, Xiaojing Ma, Yunfeng Xiao, Yufei Wang, Yuqian Bao
Obesity Facts.2020; 13(3): 358. CrossRef - Association of thyroid function with white coat hypertension and sustained hypertension
Peng Cai, Yan Peng, YuXi Chen, Li Li, Wei Chu, Yan Wang, Xukai Wang
The Journal of Clinical Hypertension.2019; 21(5): 674. CrossRef - Thyroid function is associated with body mass index and fasting plasma glucose in Thai euthyroid population
Amornpan Lertrit, La-or Chailurkit, Boonsong Ongphiphadhanakul, Wichai Aekplakorn, Chutintorn Sriphrapradang
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(1): 468. CrossRef - Thyroid disease and the metabolic syndrome
Ladan Mehran, Atieh Amouzegar, Fereidoun Azizi
Current Opinion in Endocrinology, Diabetes & Obesity.2019; 26(5): 256. CrossRef - Morbid obez hastalarda kilo kaybının insulin direnci, bazal metabolizma hızı, antropometrik ölçümler ve tiroid fonksiyonlarına etkisi
Şenay DURMAZ CEYLAN, Şuuri Ahsen CEYLAN, Fatih EKER, Aşkın GÜNGÜNEŞ
Anadolu Güncel Tıp Dergisi.2019; 1(4): 99. CrossRef - Body Composition, Resting Energy Expenditure, and Metabolic Changes in Women Diagnosed with Differentiated Thyroid Carcinoma
Elena Izkhakov, Nachum Vaisman, Sophie Barnes, Micha Barchana, Naftali Stern, Lital Keinan-Boker
Thyroid.2019; 29(8): 1044. CrossRef - High TSH Level within Normal Range Is Associated with Obesity, Dyslipidemia, Hypertension, Inflammation, Hypercoagulability, and the Metabolic Syndrome: A Novel Cardiometabolic Marker
Yi-Cheng Chang, Shih-Che Hua, Chia-Hsuin Chang, Wei-Yi Kao, Hsiao-Lin Lee, Lee-Ming Chuang, Yen-Tsung Huang, Mei-Shu Lai
Journal of Clinical Medicine.2019; 8(6): 817. CrossRef - Metabolic Syndrome, Thyroid Function and Autoimmunity - The PORMETS Study
Luís Raposo, Sandra Martins, Daniela Ferreira, João Tiago Guimarães, Ana Cristina Santos
Endocrine, Metabolic & Immune Disorders - Drug Targets.2019; 19(1): 75. CrossRef - Hormesis in Health and Chronic Diseases
Xin Li, Tingting Yang, Zheng Sun
Trends in Endocrinology & Metabolism.2019; 30(12): 944. CrossRef - Relationship of metabolic syndrome and its components with thyroid dysfunction in Algerian patients
Mohamed Larbi Hamlaoui, Ammar Ayachi, Aoulia Dekaken, Adel Gouri
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2018; 12(1): 1. CrossRef - High free triiodothyronine and free-triiodothyronine-to-free-thyroxine ratio levels are associated with metabolic syndrome in a euthyroid population
Diego Urrunaga-Pastor, Mirella Guarnizo-Poma, Enrique Moncada-Mapelli, Luis G. Aguirre, Herbert Lazaro-Alcantara, Socorro Paico-Palacios, Betzi Pantoja-Torres, Vicente A. Benites-Zapata
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2018; 12(2): 155. CrossRef - Exploring the association between thyroid- stimulating hormone and metabolic syndrome: A large population-based study
Yi-Chao Zhou, Wen-Hui Fang, Tung-Wei Kao, Chung-Ching Wang, Yaw-Wen Chang, Tao-Chun Peng, Chen-Jung Wu, Hui-Fang Yang, James Yi-Hsin Chan, Wei-Liang Chen, Tatsuo Shimosawa
PLOS ONE.2018; 13(6): e0199209. CrossRef - Thyroid function and metabolic syndrome in the population-based LifeLines cohort study
Bruce H. R. Wolffenbuttel, Hanneke J. C. M. Wouters, Sandra N. Slagter, Robert P. van Waateringe, Anneke C. Muller Kobold, Jana V. van Vliet-Ostaptchouk, Thera P. Links, Melanie M. van der Klauw
BMC Endocrine Disorders.2017;[Epub] CrossRef - Hormetic effect of triiodothyronine in metabolically healthy obese persons
Ji Eun Jun, Tae Hyuk Kim, Seung-Eun Lee, You-Bin Lee, Jae Hwan Jee, Ji Cheol Bae, Sang-Man Jin, Kyu Yeon Hur, Jae Hyeon Kim, Sun Wook Kim, Jae Hoon Chung, Yong-Ki Min, Moon-Kyu Lee
Endocrine.2017; 57(3): 418. CrossRef - Association of triiodothyronine levels with future development of metabolic syndrome in euthyroid middle-aged subjects: a 6-year retrospective longitudinal study
Hye Jeong Kim, Ji Cheol Bae, Hyeong Kyu Park, Dong Won Byun, Kyoil Suh, Myung Hi Yoo, Jee Jae Hwan, Jae Hyeon Kim, Yong-Ki Min, Sun Wook Kim, Jae Hoon Chung
European Journal of Endocrinology.2017; 176(4): 443. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- The effect of endocrine disrupting chemicals on the vitronectin-receptor (integrin αvβ3)-mediated cell adhesion of human umbilical vein endothelial cells

- Bone Metabolism
- Update on Denosumab Treatment in Postmenopausal Women with Osteoporosis
- Yong-Ki Min
- Endocrinol Metab. 2015;30(1):19-26. Published online March 27, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.1.19
- 3,814 View
- 40 Download
- 16 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Denosumab, a fully human recombinant monoclonal antibody to the receptor activator of nuclear factor-κB ligand (RANKL), blocks binding of RANKL to the RANK receptor, found on the surface of osteoclasts and osteoclast precursors, resulting in decreased bone resorption. Subcutaneous denosumab administration once every 6 months increases bone mineral density at the lumbar spine, total hip, and/or femoral neck, and reduces markers of bone turnover significantly in postmenopausal women with osteoporosis. Relative to placebo, denosumab treatment reduces the risk of vertebral, nonvertebral, and hip fractures significantly. The benefits of denosumab treatment are generally obvious after the first dose and were continued for up to 8 years of treatment in an extension study. The tolerability profile of denosumab during this extension phase was consistent with that observed during the initial 3-year FREEDOM trial. Postmarketing safety surveillance has not shown any unexpected findings. Ongoing safety surveillance will more fully define the long-term safety of denosumab. The benefits of denosumab would seem to be greater than its risks. Denosumab is an important choice in the treatment of postmenopausal women with osteoporosis at increased risk of fractures, including older patients who have difficulty with oral bisphosphonate intake and patients who are intolerant of, or unresponsive to, other therapies.
-
Citations
Citations to this article as recorded by- Comparative Pharmacokinetic Study of 5 Active Ingredients after Oral Administration of Zuogui Pill in Osteoporotic Rats with Different Syndrome Types
Jiawei Qiu, Yaoyao Zhu, Jing Xing, Ling Wang, Jianhua Zhang, Hua Yin, Suresh Ponnayyan Sulochana
International Journal of Analytical Chemistry.2023; 2023: 1. CrossRef - Benefits of lumican on human bone health: clinical evidence using bone marrow aspirates
Yun Sun Lee, So Jeong Park, Jin Young Lee, Eunah Choi, Beom-Jun Kim
The Korean Journal of Internal Medicine.2022; 37(4): 821. CrossRef - Lumican Inhibits Osteoclastogenesis and Bone Resorption by Suppressing Akt Activity
Jin-Young Lee, Da-Ae Kim, Eun-Young Kim, Eun-Ju Chang, So-Jeong Park, Beom-Jun Kim
International Journal of Molecular Sciences.2021; 22(9): 4717. CrossRef - Potential Biomarkers to Improve the Prediction of Osteoporotic Fractures
Beom-Jun Kim, Seung Hun Lee, Jung-Min Koh
Endocrinology and Metabolism.2020; 35(1): 55. CrossRef - Effect of bisphosphonate on the prevention of bone loss in patients with gastric cancer after gastrectomy: A randomized controlled trial
Jeonghoon Ha, Jung-Min Lee, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Hae Myung Jeon, Moo Il Kang, Ki-Hyun Baek
Bone.2020; 130: 115138. CrossRef - Vasomotor Symptoms: More Than Temporary Menopausal Symptoms
Ki-Jin Ryu, Hyuntae Park, Jin Seol Park, Yeon Woo Lee, Soo Young Kim, Hayun Kim, Youngmi Jeong, Yong Jin Kim, Kyong Wook Yi, Jung Ho Shin, Jun Young Hur, Tak Kim
Journal of Menopausal Medicine.2020; 26(3): 147. CrossRef - Does the OPG/RANKL system contribute to the bone-vascular axis in chronic kidney disease? A systematic review
Beata Znorko, Ewa Oksztulska-Kolanek, Małgorzata Michałowska, Tomasz Kamiński, Krystyna Pawlak
Advances in Medical Sciences.2017; 62(1): 52. CrossRef - Conservative management of osteoporotic vertebral fractures: an update
A. Slavici, M. Rauschmann, C. Fleege
European Journal of Trauma and Emergency Surgery.2017; 43(1): 19. CrossRef - Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures
Tomoko Tetsunaga, Tomonori Tetsunaga, Keiichiro Nishida, Masato Tanaka, Yoshihisa Sugimoto, Tomoyuki Takigawa, Yoshitaka Takei, Toshifumi Ozaki
Journal of Orthopaedic Science.2017; 22(2): 230. CrossRef - Safety and efficacy of denosumab in osteoporotic hemodialysed patients
Francescaromana Festuccia, Maryam Tayefeh Jafari, Alessandra Moioli, Claudia Fofi, Simona Barberi, Stefano Amendola, Salvatore Sciacchitano, Giorgio Punzo, Paolo Menè
Journal of Nephrology.2017; 30(2): 271. CrossRef - Simultaneous bilateral atypical femoral fracture in a patient receiving denosumab: case report and literature review
J. Selga, J. H. Nuñez, J. Minguell, M. Lalanza, M. Garrido
Osteoporosis International.2016; 27(2): 827. CrossRef - Vasomotor symptoms and osteoporosis in Korean postmenopausal women
Ki-Jin Ryu, Hyun-Tae Park, Yong Jin Kim, Kyong Wook Yi, Jung Ho Shin, Jun Young Hur, Tak Kim
Maturitas.2016; 87: 27. CrossRef - Osteoporosis: A Therapeutic Update
Patricia A. Mackey, Michael D. Whitaker
The Journal for Nurse Practitioners.2015; 11(10): 1011. CrossRef - Sulfonylurea: Personalized Medicine for Type 2 Diabetes
You-Cheol Hwang
Endocrinology and Metabolism.2015; 30(4): 467. CrossRef - A Systematic Review of Bone Anti-Resorptive Treatment Toxicity in Innate and Adaptive Immunity Cells: Osteonecrosis of the Jaws and Future Implications
Athanassios Kyrgidis, Maria Yavropoulou, Ioannis Tilaveridis, Charalambos Andreadis, Konstantinos Antoniades, Dimitrios Kouvelas
The Journal of Dentists.2015; 3(2): 50. CrossRef
- Comparative Pharmacokinetic Study of 5 Active Ingredients after Oral Administration of Zuogui Pill in Osteoporotic Rats with Different Syndrome Types

- Adrenal gland
- Using Growth Hormone Levels to Detect Macroadenoma in Patients with Acromegaly
- Ji Young Park, Jae Hyeon Kim, Sun Wook Kim, Jae Hoon Chung, Yong-Ki Min, Myung-Shik Lee, Moon-Kyu Lee, Kwang-Won Kim
- Endocrinol Metab. 2014;29(4):450-456. Published online December 29, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.4.450
- 3,546 View
- 29 Download
- 6 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The aim of this study was to assess the clinical differences between acromegalic patients with microadenoma and patients with macroadenoma, and to evaluate the predictive value of growth hormone (GH) levels for early detection of macroadenoma.
Methods We performed a retrospective analysis of 215 patients diagnosed with a GH-secreting pituitary adenoma. The patients were divided into two groups: the microadenoma group and the macroadenoma group, and the clinical parameters were compared between these two groups. The most sensitive and specific GH values for predicting macroadenoma were selected using receiver operating characteristic (ROC) curves.
Results Compared with the microadenoma group, the macroadenoma group had a significantly younger age, higher body mass index, higher prevalence of hyperprolactinemia and hypogonadism, and a lower proportion of positive suppression to octreotide. However, there were no significant differences in the gender or in the prevalence of diabetes between the two groups. The tumor diameter was positively correlated with all GH values during the oral glucose tolerance test (OGTT). All GH values were significantly higher in the macroadenoma group than the microadenoma group. Cut-off values for GH levels at 0, 30, 60, 90, and 120 minutes for optimal discrimination between macroadenoma and microadenoma were 5.6, 5.7, 6.3, 6.0, and 5.8 ng/mL, respectively. ROC curve analysis revealed that the GH value at 30 minutes had the highest area under the curve.
Conclusion The GH level of 5.7 ng/mL or higher at 30 minutes during OGTT could provide sufficient information to detect macroadenoma at the time of diagnosis.
-
Citations
Citations to this article as recorded by- Sex differences in acromegaly at diagnosis: A nationwide cohort study and meta‐analysis of the literature
Jakob Dal, Benedikte G. Skov, Marianne Andersen, Ulla Feldt‐Rasmussen, Claus L. Feltoft, Jesper Karmisholt, Eigil H. Nielsen, Olaf M. Dekkers, Jens Otto L. Jørgensen
Clinical Endocrinology.2021; 94(4): 625. CrossRef - Pretreatment serum GH levels and cardio-metabolic comorbidities in acromegaly; analysis of data from Iran Pituitary Tumor Registry
Leila Hedayati Zafarghandi, Mohammad Ebrahim Khamseh, Milad Fooladgar, Shahrzad Mohseni, Mostafa Qorbani, Nahid Hashemi Madani, Mahboobeh Hemmatabadi, MohammadReza Mohajeri-Tehrani, Nooshin Shirzad
Journal of Diabetes & Metabolic Disorders.2020; 19(1): 319. CrossRef - Increased serum nesfatin-1 levels in patients with acromegaly
Yakun Yang, Song Han, Zuocheng Yang, Pengfei Wang, Chang-Xiang Yan, Ning Liu
Medicine.2020; 99(40): e22432. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef
- Sex differences in acromegaly at diagnosis: A nationwide cohort study and meta‐analysis of the literature

- Bone Metabolism
- Efficacy of a Once-Monthly Pill Containing Ibandronate and Cholecalciferol on the Levels of 25-Hydroxyvitamin D and Bone Markers in Postmenopausal Women with Osteoporosis
- In-Jin Cho, Ho-Yeon Chung, Sung-Woon Kim, Jae-Won Lee, Tae-Won Lee, Hye-Soon Kim, Sin-Gon Kim, Han Seok Choi, Sung-Hee Choi, Chan Soo Shin, Ki-Won Oh, Yong-Ki Min, Jung-Min Koh, Yumie Rhee, Dong-Won Byun, Yoon-Sok Chung, Jeong Hyun Park, Dong Jin Chung, Minho Shong, Eun-Gyoung Hong, Chang Beom Lee, Ki Hyun Baek, Moo-Il Kang
- Endocrinol Metab. 2015;30(3):272-279. Published online December 9, 2014
- DOI: https://doi.org/10.3803/EnM.2015.30.3.272
- 4,511 View
- 47 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The present study evaluated the efficacy of a combination of ibandronate and cholecalciferol on the restoration of the levels of 25-hydroxyvitamin D (25[OH]D) and various bone markers in postmenopausal women with osteoporosis.
Methods This was a randomized, double-blind, active-controlled, prospective 16-week clinical trial conducted in 20 different hospitals. A total of 201 postmenopausal women with osteoporosis were assigned randomly to one of two groups: the IBN group, which received a once-monthly pill containing 150 mg ibandronate (
n =99), or the IBN+ group, which received a once-monthly pill containing 150 mg ibandronate and 24,000 IU cholecalciferol (n =102). Serum levels of 25(OH)D, parathyroid hormone (PTH), and various bone markers were assessed at baseline and at the end of a 16-week treatment period.Results After 16 weeks of treatment, the mean serum levels of 25(OH)D significantly increased from 21.0 to 25.3 ng/mL in the IBN+ group but significantly decreased from 20.6 to 17.4 ng/mL in the IBN group. Additionally, both groups exhibited significant increases in mean serum levels of PTH but significant decreases in serum levels of bone-specific alkaline phosphatase and C-telopeptide of type 1 collagen (CTX) at 16 weeks; no significant differences were observed between the groups. However, in subjects with a vitamin D deficiency, IBN+ treatment resulted in a significant decrease in serum CTX levels compared with IBN treatment.
Conclusion The present findings demonstrate that a once-monthly pill containing ibandronate and cholecalciferol may be useful for the amelioration of vitamin D deficiency in patients with postmenopausal osteoporosis. Moreover, this treatment combination effectively decreased serum levels of resorption markers, especially in subjects with a vitamin D deficiency, over the 16-week treatment period.
-
Citations
Citations to this article as recorded by- Effect of vitamin D supplementation or fortification on bone turnover markers in women: a systematic review and meta-analysis
Nasrin Nasimi, Sanaz Jamshidi, Aida Askari, Nazanin Zolfaghari, Erfan Sadeghi, Mehran Nouri, Nick Bellissimo, Shiva Faghih
British Journal of Nutrition.2024; 131(9): 1473. CrossRef - Quality of life and patient satisfaction with raloxifene/cholecalciferol combination therapy in postmenopausal women
Dong-Yun Lee, Yoon-Sok Chung
Scientific Reports.2022;[Epub] CrossRef - Efficacy of risedronate with cholecalciferol on bone mineral density in Korean patients with osteoporosis
So Young Park, Moo-Il Kang, Hyung Moo Park, Yumie Rhee, Seong Hwan Moon, Hyun Koo Yoon, Jung-Min Koh, Jae Suk Chang, In Joo Kim, Ye Yeon Won, Ye Soo Park, Hoon Choi, Chan Soo Shin, Taek Rim Yoon, Sung-Cheol Yun, Ho-Yeon Chung
Archives of Osteoporosis.2020;[Epub] CrossRef - Efficacy and safety of vitamin D3 B.O.N intramuscular injection in Korean adults with vitamin D deficiency
Han Seok Choi, Yoon-Sok Chung, Yong Jun Choi, Da Hea Seo, Sung-Kil Lim
Osteoporosis and Sarcopenia.2016; 2(4): 228. CrossRef - Pharmacologic treatment of osteoporosis
Yong-Ki Min
Journal of the Korean Medical Association.2016; 59(11): 847. CrossRef
- Effect of vitamin D supplementation or fortification on bone turnover markers in women: a systematic review and meta-analysis


 KES
KES

 First
First Prev
Prev



